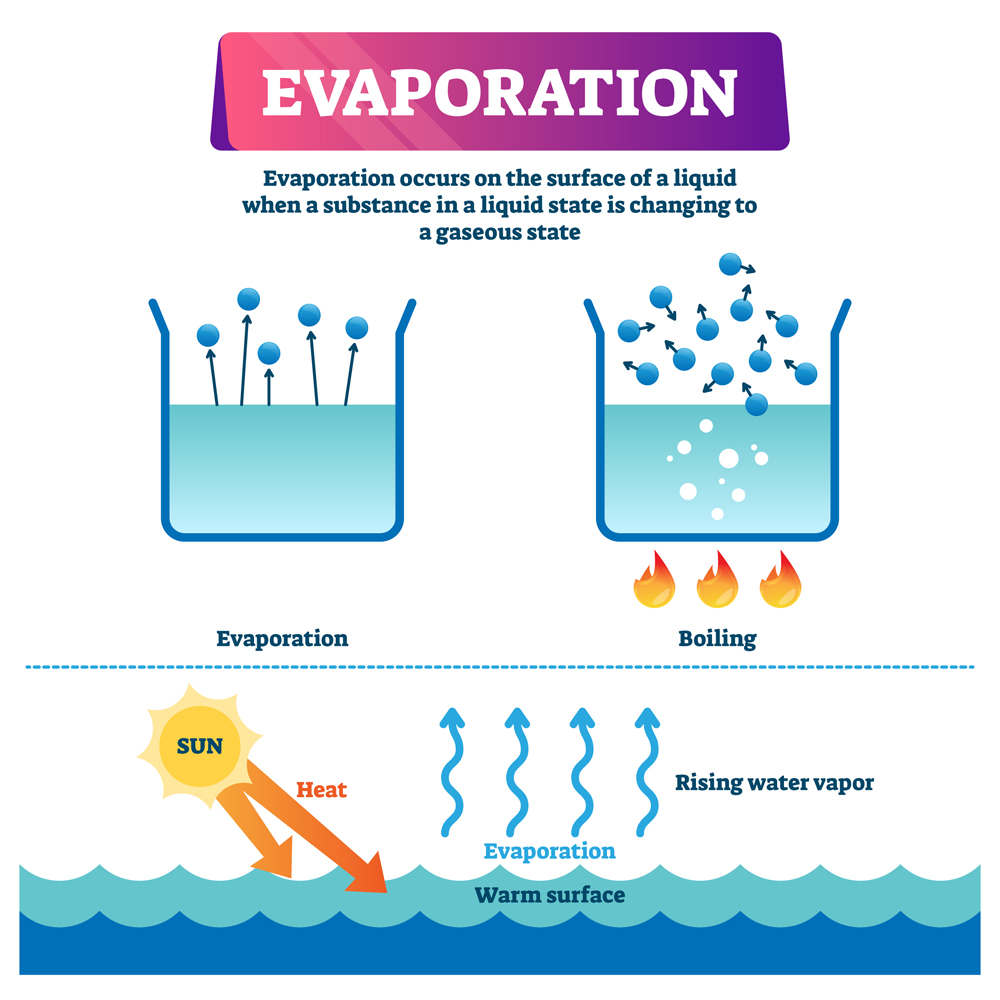
What Does Evaporating Temperature Mean . When you spray perfume on your body, your body. It can be easily visualized when rain puddles “disappear” on a. evaporation happens when a liquid turns into a gas. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. evaporation can generally be defined as a process by which a liquid is transformed into vapour. For evaporation to occur, molecules in a liquid must be near the surface, must. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state. evaporation is the opposite of condensation. When you’re boiling water on the stove, you’re. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break.
from www.scienceabc.com
When you’re boiling water on the stove, you’re. For evaporation to occur, molecules in a liquid must be near the surface, must. evaporation can generally be defined as a process by which a liquid is transformed into vapour. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. When you spray perfume on your body, your body. It can be easily visualized when rain puddles “disappear” on a. evaporation happens when a liquid turns into a gas. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. evaporation is the opposite of condensation. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state.
Why Does Water Evaporate At Room Temperature?
What Does Evaporating Temperature Mean the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. evaporation can generally be defined as a process by which a liquid is transformed into vapour. When you spray perfume on your body, your body. When you’re boiling water on the stove, you’re. It can be easily visualized when rain puddles “disappear” on a. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state. evaporation happens when a liquid turns into a gas. evaporation is the opposite of condensation. For evaporation to occur, molecules in a liquid must be near the surface, must. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break.
From www.slideserve.com
PPT Freezing, Melting, and Evaporation PowerPoint Presentation, free What Does Evaporating Temperature Mean evaporation happens when a liquid turns into a gas. When you spray perfume on your body, your body. When you’re boiling water on the stove, you’re. It can be easily visualized when rain puddles “disappear” on a. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. evaporation is. What Does Evaporating Temperature Mean.
From dxowlbddi.blob.core.windows.net
Explain The Evaporation Process at James Ligon blog What Does Evaporating Temperature Mean It can be easily visualized when rain puddles “disappear” on a. evaporation happens when a liquid turns into a gas. When you’re boiling water on the stove, you’re. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. evaporation occurs when energy (heat) forces the bonds that hold water. What Does Evaporating Temperature Mean.
From www.researchgate.net
Compressor power consumption vs. evaporating temperature. Base What Does Evaporating Temperature Mean When you spray perfume on your body, your body. It can be easily visualized when rain puddles “disappear” on a. evaporation is the opposite of condensation. When you’re boiling water on the stove, you’re. For evaporation to occur, molecules in a liquid must be near the surface, must. evaporation, process by which an element or compound transitions from. What Does Evaporating Temperature Mean.
From www.researchgate.net
Total exergy efficiency versus evaporating temperature. Download What Does Evaporating Temperature Mean evaporation can generally be defined as a process by which a liquid is transformed into vapour. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. When you’re boiling water on the stove, you’re. When you spray perfume on your body, your body. areas with. What Does Evaporating Temperature Mean.
From www.researchgate.net
Effects of evaporating temperature on total condensing heat. Download What Does Evaporating Temperature Mean evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. It can be easily visualized when rain puddles “disappear” on a. When you’re boiling water on the stove, you’re. evaporation,. What Does Evaporating Temperature Mean.
From www.researchgate.net
Effect of the evaporating temperature on the system's pressure ratio What Does Evaporating Temperature Mean evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. When you’re. What Does Evaporating Temperature Mean.
From www.youtube.com
Lecture 4 Introduction to evaporation and latent heat YouTube What Does Evaporating Temperature Mean the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. evaporation is the opposite of condensation. When you’re boiling water on the stove, you’re. It can be easily visualized when. What Does Evaporating Temperature Mean.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature What Does Evaporating Temperature Mean When you’re boiling water on the stove, you’re. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. It can be easily visualized when rain puddles “disappear” on a. evaporation can generally be defined as a process by which a liquid is transformed into vapour. For. What Does Evaporating Temperature Mean.
From www.researchgate.net
Influence of evaporating temperature (VC cycle condensing temperature What Does Evaporating Temperature Mean evaporation happens when a liquid turns into a gas. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. It can be easily visualized when rain puddles. What Does Evaporating Temperature Mean.
From sciencenotes.org
Evaporative Cooler How It Works and Examples What Does Evaporating Temperature Mean areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. When you spray perfume on your body, your body. evaporation happens when a liquid turns into a gas. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules. What Does Evaporating Temperature Mean.
From www.researchgate.net
Discharge temperature versus evaporating temperature. Download What Does Evaporating Temperature Mean evaporation, process by which an element or compound transitions from its liquid state to its gaseous state. evaporation happens when a liquid turns into a gas. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that. When you’re boiling water on the stove, you’re. . What Does Evaporating Temperature Mean.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? What Does Evaporating Temperature Mean For evaporation to occur, molecules in a liquid must be near the surface, must. It can be easily visualized when rain puddles “disappear” on a. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. evaporation happens when a liquid turns into a gas. evaporation can generally be defined. What Does Evaporating Temperature Mean.
From www.researchgate.net
Heat pump evaporating temperature during frost buildup with the base What Does Evaporating Temperature Mean evaporation happens when a liquid turns into a gas. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. evaporation can generally be defined as a process by which a liquid is transformed into vapour. the liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the. What Does Evaporating Temperature Mean.
From www.researchgate.net
Variation of evaporating temperature with refrigerant charge ratio and What Does Evaporating Temperature Mean evaporation can generally be defined as a process by which a liquid is transformed into vapour. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. When you’re boiling water on the stove, you’re. It can be easily visualized when rain puddles “disappear” on a. evaporation happens when a. What Does Evaporating Temperature Mean.
From www.researchgate.net
Average temperature differences between the evaporating temperature and What Does Evaporating Temperature Mean For evaporation to occur, molecules in a liquid must be near the surface, must. evaporation happens when a liquid turns into a gas. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. . What Does Evaporating Temperature Mean.
From www.researchgate.net
Evaporating Pressure Vs evaporating temperature. Download Scientific What Does Evaporating Temperature Mean It can be easily visualized when rain puddles “disappear” on a. areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. When you’re boiling water on the stove, you’re. For evaporation to occur, molecules in a liquid must be near the surface, must. When you spray perfume on your body, your. What Does Evaporating Temperature Mean.
From www.chegg.com
Solved The evaporating and condensing temperatures of a What Does Evaporating Temperature Mean evaporation can generally be defined as a process by which a liquid is transformed into vapour. When you’re boiling water on the stove, you’re. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. When. What Does Evaporating Temperature Mean.
From mavink.com
Water Evaporation Chart What Does Evaporating Temperature Mean evaporation, process by which an element or compound transitions from its liquid state to its gaseous state. It can be easily visualized when rain puddles “disappear” on a. evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. areas with high temperatures and large bodies of water, such as tropical islands and. What Does Evaporating Temperature Mean.